Microbiology: Bacteria Perform Self-maintenance
Certain bacteria form complex molecular structures that function like tiny machines and resemble needles. These structures assist in invading host cells by injecting proteins into them – an important step when it comes to infections. Researchers at Karlsruhe Institute of Technology (KIT) have now shown that in Yersinia enterocolitica, a bacterium related to the plague pathogen, the inner membrane ring of the so-called type III secretion system (T3SS) is continuously renewed. This renewal is essential for the assembly and operation of the injection apparatus. The findings offer potential for the development of antimicrobial therapies.
New dynamics in the injection apparatus
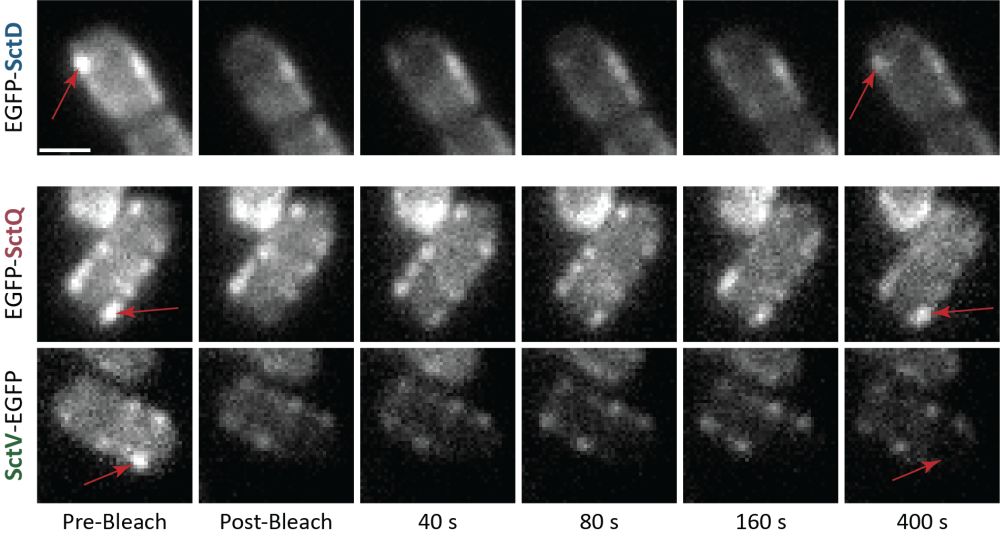
The team led by Dr. Andreas Diepold from the Institute of Applied Biosciences at KIT investigated the inner membrane protein SctD and discovered that its subunits are replaced even in fully assembled T3SS structures. “The replacement of the ring components is central to the function of the secretion system,” explains Diepold.
The fact that even core structural elements are dynamically exchanged makes the system adaptable and persistently functional—an especially relevant feature in the context of infection. At the same time, this dynamic behavior opens up new points of intervention: disrupting the exchange process can inhibit secretion. “This could represent a potential approach for antimicrobial strategies,” says Diepold. The T3SS can also be used as a tool for the targeted delivery of proteins.
In future research, the KIT team aims to determine which molecular mechanisms regulate the exchange of SctD and how environmental stimuli influence this process. “We are at the beginning of a new understanding of how bacterial nanomachines are not only assembled but also remain mobile and adaptable,” says Diepold.
mfe, November 20, 2025
Photo:
To demonstrate the exchange of SctD (top), the researchers labeled a variant of the protein (red arrow). The reappearance of the label indicates that the protein has been replaced—similar to the dynamic protein SctQ (middle), but unlike the stably integrated SctV (bottom).