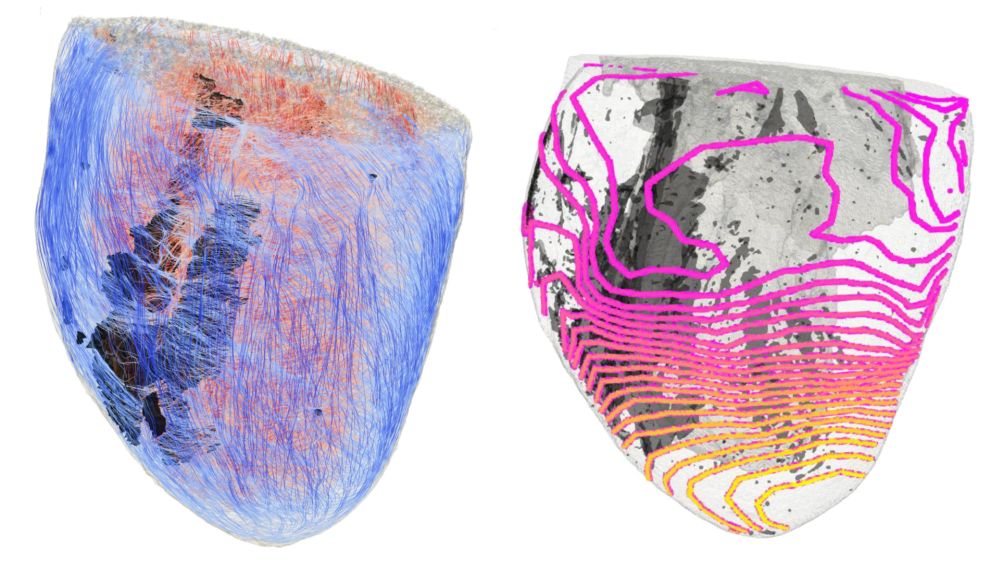
How Fibrosis Disrupts the Heart's Electrical Signals
Cardiac fibrosis can be life-threatening: the formation of connective tissue in the heart muscle causes it to become stiffer, impairs electrical signal transmission, and weakens pumping capacity, which can lead to cardiac arrhythmias. However, the effects of cardiac fibrosis on the electrical conductivity of the heart have been insufficiently studied to date. A research team led by the University of Freiburg, with the participation of the Karlsruhe Institute of Technology (KIT), has now closed this gap, gaining new insights into the fundamentals of electrophysiology in cardiac fibrosis and thus opening up further possibilities for personalized diagnostic and therapeutic approaches.
Computer models and digital twins
Using optical imaging and 3D computer models of mouse hearts, the researchers found that fibrotic tissue acts as a “low-pass filter” for electrical activation of the heart, the electrophysiology: The slowed or blocked transmission of electrical impulses at a higher pulse rate, for example due to physical exertion, can lead to dangerous cardiac arrhythmias – as is the case with arrhythmogenic cardiomyopathy, which occurs particularly in young people. Targeted stress tests are therefore important for the diagnosis of high-risk patients.
“For the first time, we were able to decipher the influence of connective tissue, interacting with the muscle cells of the heart, in arrhythmogenic cardiomyopathy”, explains Dr. Francesco Giardini from the University of Freiburg. The “Computer Models of the Heart” research group led by Dr.Axel Loewe from KIT has integrated the experimental data in computer simulations and developed a digital twin of the biological experiment, showing the disrupted propagation of excitation at higher heart rates. “We want to extend the method to larger animal models and models of the human heart in order to further develop digital twins for precision cardiology and thus improve clinical diagnostics and treatment,” says Loewe.
lkr, October 7, 2025