|
Dr. Elisabeth Zuber-Knost Kaiserstraße 12 | ||
|
Reactions with oxygen are among the most important chemical processes. They are useful during the controlled burning of food in body cells or of natural gas in power plants to carbon dioxide in order to generate energy. However, they may become an ecological problem when, together with air oxygen, ozone or nitrogen oxides are formed. Oxygen-dependent corrosion of iron, i.e. rust, is of particular economic and technical relevance. Although very widespread, little is known about the atomic processes of oxidation with oxygen. An article published by researchers from the Karlsruhe Institute of Technology (KIT) in the journal “Science” now brings light into the darkness. |
For details, please contact: Universität Karlsruhe (TH)
Dr. Gerd König Wolfgang-Gaede-Str. 1 | |
|
According to the publication by the chemists Professor Hansgeorg Schnöckel and Dr. Ralf Burgert, the spin, i.e. the angular momentum of the electrons which are responsible for the bonding between atoms, is of decisive importance in the corrosion of metals (Science, 319 (5862), 438). As a model system, the scientists produced nanoparticles consisting of only a few aluminum atoms. To study these so-called clusters, they used the Fourier transform mass spectrometry, a technique that has been applied mainly for the analysis of proteins so far. In cooperation with American scientists from Baltimore, Lake Charles, and Richmond as well as with researchers from the University of Constance, the chemists could show that the spin determines whether a spontaneous oxidation takes place or not. It is important that the spins of both reaction partners fit to each other, because certain combinations only are possible in accordance with the “spin selection rules”. “In fact, everything around us should burn, because our air contains 20 percent oxygen,” explains Schnöckel, who works at the Center for Functional Nanostructures of the KIT. “Fortunately, however, oxygen exists in various quantum mechanical forms. Due to the electron states, it has both the lowest energy and magnetic properties in the form of triplet oxygen, i.e. in its ‘normal’ form. Singlet oxygen is generated only if energy is supplied, e.g. by UV radiation in the upper layers of the atmosphere or by chemical reactions at the laboratory. It is not magnetic and highly unstable due to its higher energy.” Schnöckel’s team found that this form exclusively oxidizes a highly stable cluster of 13 aluminum atoms (Al13) without supplying energy. For this purpose, they caught negatively charged Al13 clusters in the magnetic field of a mass spectrometer on a circular path. By means of electric discharges, they produced singlet oxygen that is stable for a longer term in the high vacuum of the system. As the reaction partners exist in small concentrations only, a molecule hits an aluminum cluster about every 10 seconds only - sufficient time to measure the quick first reaction step of oxidation. In the same experiment, a cluster of 14 aluminum atoms, which itself acts like a minute magnet, reacted not with singlet, but with triplet oxygen. So far, the team has studied small aluminum clusters exclusively. According to Schnöckel, their regular structures make them ideal experimental objects. “In simple words, Al13 clusters behave like aluminum metal.” His studies and methods may also be transferred to other reactions with oxygen, such as those taking place during combustion or the decomposition of plastics. This may help to better understand and control these reactions. Literature: Spin Conservation Accounts for Aluminum Cluster Anion Reactivity Pattern with O2. R. Burgert et al., Science 319 (5862), 438-442 (2008). | ||
|
|
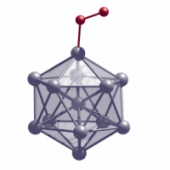
A negatively charged Al13 cluster with its stable electron structure does only slowly react with an oxygen molecule in the triplet state (red). Due to the rotation direction of its electrons (spin), the molecule behaves like a small magnet and shows restricted reactivity. | |
|
|
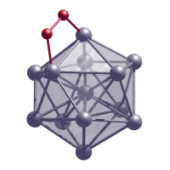
The electron spin of the non-magnetic singlet oxygen molecule (red) allows for rapid bonding to the negatively charged Al13 ion. In an intermediate step, the cluster is deformed. In the end of the oxidation reaction, it decomposes into two molecules of aluminum oxide and an unstable Al9 ion. | |
|
Photos by: CFN, Universität Karlsruhe (TH) |
The mass spectrometer funded by the Deutsche Forschungsgemeinschaft (German Research Foundation) costs one million Euros. It is used by the Karlsruhe chemists to study the reaction of aluminum nanoparticles with oxygen. | |
|
| ||
|
The Karlsruhe Institute of Technology (KIT) represents the merger of the Universität Karlsruhe with the Forschungszentrum Karlsruhe. Altogether, it has 8000 employees and an annual budget of 600 million Euros. The KIT will be an institution of internationally excellent research and teaching in natural and engineering sciences. KIT shall attract the best experts from all over the world, set new standards in teaching and promotion of young scientists, and establish the leading European center in the field of energy research. KIT will assume a leading role in nanosciences worldwide. It is the objective of KIT to be one of the most important cooperation partners of industry. Background Information Oxidation Colloquially speaking, oxidation is a reaction with oxygen. For a chemist the term oxidation refers to any reaction, during which a substance donates electrons to an electron acceptor that is reduced. As both steps do not occur independently of each other, it is referred to redox reactions. Hence, there are elements/compounds that preferably donate electrons (e.g. metals) and elements/compounds that preferably accept electrons (e.g. oxygen, chlorine). The latter are referred to as oxidants, the former as reductants. Spin Selection Rules, Spin Conservation Law For spontaneous reactions, the total spin (angular momentum of electrons) of the initial compounds must be equal to the total spin of the reaction product. This spin conservation law is violated for reactions with triplet oxygen, because most products, such as CO2 in the combustion of carbon, do not possess any spin, i.e. the total angular momentum is zero. Mass Spectrometer Using a mass spectrometer, it is possible to measure the ratio between the mass and charge of particles. Ionized (electrically charged) atoms and compounds are accelerated and exposed to a magnetic field. Depending on their mass, they are deflected from their trajectory more or less strongly. If the charge is known, the mass of the particle can be calculated from the deflection. | ||
Press Release 04/2008
Spin Adds New Twist to Oxidation Research
Quantum Mechanics Determines the Reaction of Oxygen with Metals
January 30, 2008
Contact:
Christian Könemann
Chief Press Officer
Phone: +49 721 608-41190
Fax: +49 721 608-43658
christian koenemann ∂does-not-exist.kit edu