The behavior of nanoparticles in the body does not only depend on their chemical composition. It is of decisive importance how they interact with biological molecules. Professor Gerd Ulrich Nienhaus from the Karlsruhe Institute of Technology (KIT) has developed new methods to quantitatively determine this dynamic process (Nature Nanotechnology 4, 577 (2009)).
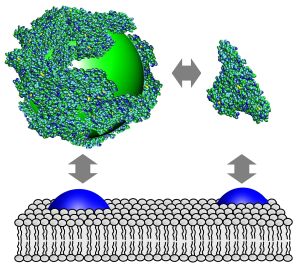
If a nanoparticle enters the blood, it is immediately enclosed by a thin layer of biomolecules. This biological surface coating that is also referred to as protein corona determines decisively whether the particle is simply excreted or may enter the interior of a body cell. “Nanoparticles that erroneously enter the body should preferably be eliminated from the body quickly. But if they are used for therapeutic purposes, for example, it is desirable that they are selectively taken up by certain cell types. Therefore, it is important to understand how the body’s own molecules bind to nanoparticles, because a nanoparticle establishes contact with a cell surface via the biomolecule layer,” explains Nienhaus, who recently moved to the KIT Center for Functional Nanostructures from the University of Ulm. His analytical methods published in the renowned journal Nature Nanotechnology allow for an experimental investigation of these issues.
As model protein, the biophysicist chose serum albumin, an important blood protein. If it attaches to the surface of a nanoparticle, the latter’s diameter increases. In an aqueous solution, nanoparticles move constantly. This diffusion movement slows down with increasing particle size. To determine how thick the protein layer on a nanoparticle is, Nienhaus and his team measure the time that is needed by the particle to move through a minute liquid volume.
Nanoparticles are produced such that they emit fluorescent light when they are exposed to light. For this reason, they can be observed in spite of their small diameter of 6 to 8 nm only (1 nanometer = 1 millionth of a millimeter). If a particle passes an extremely small liquid volume in the test chamber of a specially designed microscope, it is hit by a laser beam and emits light for a fraction of a second. The length of this light flash can be measured precisely. If the flash is short, the particle moves quickly, if it is long, the particle moves slowly, which suggests that it has a larger diameter. “As we know the size of an albumin molecule, the total particle size can be calculated with the help of known physical formulas. Accordingly, the protein layer on a nanoparticle has a thickness of one molecule layer only,” summarizes Nienhaus the results.
But how quickly does this coating build up and how stable is it? To answer this question, the researchers mark the proteins with a dye that quenches the fluorescence of the nanoparticle. If the so treated protein molecules bind to a particle, the latter’s fluorescence intensity is reduced. Measurement data show that a serum albumin molecule adheres to the particle surface for about 100 s on average, before it is detached again and replaced by another one.
Nienhaus and his team now want to study further combinations of various biomolecules and nanoparticles. Experiments with cell cultures will be conducted to find out how cells react to the coated nanoparticles. The methods developed by the scientist from Karlsruhe open up new measurement options that will also be essential in evaluating the risk of nanoparticles – an aspect that is emphasized by the article published on the research project of Nienhaus and his team under “News and Views” in the Nature Nanotechnology journal.
Literature:
A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Carlheinz Röcker, Matthias Pötzl, Feng Zhang, Wolfgang J. Parak, and G. Ulrich Nienhaus. Nature Nanotechnology 4, 577 (2009).
What does the cell see? Iseult Lynch, Anna Salvati, and Kenneth A. Dawson. (News & Views) Nature Nanotechnology 4, 546 (2009).
Background Information:
Fluorescence Correlation Spectroscopy
Fluorescence correlation spectroscopy, FCS, is used to study dynamic properties of particles and individual molecules in a solution. For this purpose, photons emitted by fluorescent objects upon excitation with laser light are recorded as a function of time using a confocal microscope. As the laser beam is extremely focused, the sample volume studied amounts to about 1 femtoliter only (1 femtoliter = 1 billionth of a liter). The fluorescence intensity measured (number of photons per time interval) fluctuates, as single marked molecules diffuse into and out of the sample volume due to Brownian molecular motion, or light emission is varied by chemical or physical modifications of the molecule. Statistical analysis of these fluctuations allows for a precise determination of diffusion constants and relaxation times.
In close partnership with society, KIT develops solutions for urgent challenges – from climate change, energy transition and sustainable use of natural resources to artificial intelligence, sovereignty and an aging population. As The University in the Helmholtz Association, KIT unites scientific excellence from insight to application-driven research under one roof – and is thus in a unique position to drive this transformation. As a University of Excellence, KIT offers its more than 10,000 employees and 22,800 students outstanding opportunities to shape a sustainable and resilient future. KIT – Science for Impact.