KIT researchers have developed a new concept for rechargeable batteries. Based on a fluoride shuttle - the transfer of fluoride anions between the electrodes – it promises to enhance the storage capacity reached by lithium-ion batteries by several factors. Operational safety is also increased, as it can be done without lithium. The fluoride-ion battery is presented for the first time in the “Journal of Materials Chemistry” by Dr. Maximilian Fichtner and Dr. Munnangi Anji Reddy.
Lithium-ion batteries are applied widely, but their storage capacity is limited. In the future, battery systems of enhanced energy density will be needed for mobile applications in particular. Such batteries can store more energy at reduced weight. For this reason, KIT researchers are also conducting research into alternative systems. A completely new concept for secondary batteries based on metal fluorides was developed by Dr. Maximilian Fichtner, Head of the Energy Storage Systems Group, and Dr. Munnangi Anji Reddy at the KIT Institute of Nanotechnology (INT).
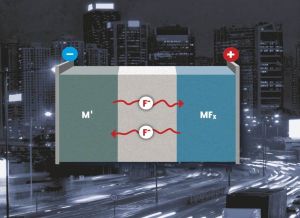
Metal fluorides may be applied as conversion materials in lithium-ion batteries. They also allow for lithium-free batteries with a fluoride-containing electrolyte, a metal anode, and metal fluoride cathode, which reach a much better storage capacity and possess improved safety properties. Instead of the lithium cation, the fluoride anion takes over charge transfer. At the cathode and anode, a metal fluoride is formed or reduced. “As several electrons per metal atom can be transferred, this concept allows to reach extraordinarily high energy densities – up to ten times as high as those of conventional lithium-ion batteries,” explains Dr. Maximilian Fichtner.
The KIT researchers are now working on the further development of material design and battery architecture in order to improve the initial capacity and cyclic stability of the fluoride-ion battery. Another challenge lies in the further development of the electrolyte: The solid electrolyte applied so far is suited for applications at elevated temperatures only. It is therefore aimed at finding a liquid electrolyte that is suited for use at room temperature.
M. Anji Reddy and M. Fichtner: Batteries based on fluoride shuttle. Journal of Materials Chemistry. 2011, Advance Article. DOI: 10.1039/C1JM13535J.
In close partnership with society, KIT develops solutions for urgent challenges – from climate change, energy transition and sustainable use of natural resources to artificial intelligence, sovereignty and an aging population. As The University in the Helmholtz Association, KIT unites scientific excellence from insight to application-driven research under one roof – and is thus in a unique position to drive this transformation. As a University of Excellence, KIT offers its more than 10,000 employees and 22,800 students outstanding opportunities to shape a sustainable and resilient future. KIT – Science for Impact.