|
Dr. Elisabeth Zuber-Knost Kaiserstraße 12 | |
|
“Take tin chloride and indium chloride, put them in a pot filled with ionic liquid, and heat it in a microwave.” This is not the latest creation of kitchen chemistry, but a new process for the rapid and simple synthesis of electrically conducting indium tin oxide (ITO) Nanoparticles. Without any complex intermediate steps, Professor Claus Feldmann from the Karlsruhe Institute of Technology (KIT) uses this process to generate uniform and regular crystals of 10 to 15 nm (1 nm = 1 millionth of a millimeter) in size, which do not clump together and can be dispersed easily in aqueous media. By means of conventional techniques, these nanocrystals may be printed as invisible electrodes onto transparent, flexible or heat-sensitive materials. Using the meanwhile patented “one-pot microwave synthesis” in ionic liquids, however, Feldmann also produces other nanoscaled particles, such as luminescent materials that are transparent in visible light, but glow in various colors under UV light. |
For details, please contact: DFG-Centrum für Funktionelle Nanostrukturen (CFN, Center for Functional Nanostructures)
Dr. Gerd König |
|
Nanoparticles that may be applied as transparent, electrically conductive or luminescent layers of a few nanometers in thickness are used in light-emitting diodes and solar cells, for security labeling or decorative purposes. To obtain highly uniform crystals without defects in their lattice structure, high temperatures (up to 600°C) are usually required. Specifically added substances that enclose the newly formed particles like a nutshell may prevent these particles from clumping together in larger aggregates. “But synthesis is highly elaborate, and some additives are toxic. Nanoparticles for therapeutic or diagnostic applications in medicine can hardly be synthesized by this process,” explains Feldmann. To avoid these drawbacks, the chemist from the DFG-Center for Functional Nanostructures of the KIT uses so-called ionic liquids as solvents. They exclusively consist of large cations and anions and, hence, represent a water-free, non-crystalline salt. At temperatures ranging from -50 to +400°C, they are liquid and chemically stable. As they hardly interact with the dissolved particles, they can be removed easily during purification of the products. However, this property has a catch: newly formed particles are not enclosed by a protective coat of solvent molecules that prevents their contacting one another. When the mixture is heated in a conventional way, larger complexes form due to the temperature gradient within the solution, which cannot be dissociated afterwards. This is where the microwave comes into play: within seconds it homogeneously heats the sample in the vessel, and aggregation of the particles is prevented. “Our first experiments were indeed carried out in a simple household device,” Feldmann recalls. Since then, he uses a special laboratory microwave, in which the reaction solution can be stirred and its temperature be measured. But the industrial use of his synthesis process is still far ahead. Ionic liquids that have hardly been exploited technically so far are relatively expensive. However, Feldmann is convinced that the costs will decrease with increasing demand. Moreover, the liquid salts may be reused after synthesis. Chemical companies like Evonik Degussa GmbH have high hopes for this new method and cooperate closely with the chemist from Karlsruhe, whose work receives additional funds from the states of Baden-Württemberg and Northrhine-Westphalia, the European Union, and the Deutsche Forschungsgemeinschaft (German Research Foundation). Literature: One-pot Synthesis of Highly Conductive ITO Nanocrystals. G. Bühler, D. Thölmann, C. Feldmann, Adv. Mater. 19, 2224 (2007). Mikrowellen-unterstützte Synthese lumineszierender LaPO4:Ce,Tb-Nanokristalle in Ionischen Flüssigkeiten. G. Bühler, C. Feldmann, Angew. Chem. 118, 4982 (2006). The Karlsruhe Institute of Technology (KIT) represents the merger of the Universität Karlsruhe with the Forschungszentrum Karlsruhe. Altogether, it has 8000 employees and an annual budget of 600 million Euros. In the KIT, both partners are bundling their scientific competences and capacities, establishing optimum research structures, and developing joint strategies and visions. The KIT will be an institution of internationally excellent research and lecturing in natural and engineering sciences. KIT shall attract the best experts from all over the world, set new standards in lecturing and promotion of young scientists, and establish the leading European center in the field of energy research. KIT will assume a leading role in nanosciences worldwide. It is the objective of KIT to be one of the most important cooperation partners of industry. Dr. Gerd König | |
Press Release 16/2007
Nanocrystals from the Microwave
Scientists from Karlsruhe Use Ionic Liquids and Microwaves to Produce Nanoparticles
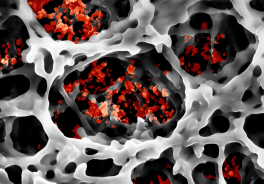
Smallest particles in a network – paper fibers with luminescent nanoparticles. Photo: CFN
November 05, 2007
Contact:
Monika Landgraf
Chief Communication Officer
Head of Corporate Communications
Chief Press Officer
Phone: +49 721 608-41150
Fax: +49 721 608-43658
presse ∂does-not-exist.kit edu
The photo in the best quality available to us may be requested by
presse ∂does-not-exist.kit edu or phone: +49 721 608-41105.
presse ∂does-not-exist.kit edu or phone: +49 721 608-41105.

